
Original manuscript by S.C. Myers and A.J. Lewis, former Extension horticulturists; Reviewed/revised by Bodie V. Pennisi, Gary L. Wade, Melvin P. Garber, Paul A. Thomas and James T. Midcap, Department of Horticulture
- Equivalents for liquid measure (volume)
- Equivalents for dry measure and weight
- Metric system conversion table
- Dilution of liquid pesticides at various concentrations
- Equivalent quantities of dry materials (wettable powders) for various quantities of water based on recommended pounds per 100 gallons
- Equivalent quantities of liquid materials (emulsion concentrates, etc.) for various quantities of water based on pints per 100 gallons
- Rate of application equivalent table
- Fertilizer conversions for specified square feet and row areas
- Fertilizer weight as measured by standard pot size
- Element concentrations for pounds soluble fertilizer in 1000 gallons (U.S.) water
- Injection ratios and nitrogen concentrations for constant fertilization
- Injector calibration with a conductivity meter
- Parts per million of desired nutrient to ounces of fertilizer carrier in 100 gallons of water (or grams in 1 liter) and vice versa
- Conversion factors among electrical conductivity (EC) units
- Various acids to add to irrigation water for acidification
- Amounts of nutrient sources to combine in making various fertilizer formulas
- Formulas for additional fertilizer calculations
- Miscellaneous conversions used in fertilizer calculations
- Osmocote® controlled-release fertilizers and their release periods
- Rates in lb/yd3 (kg/m3) for incorporation of three of the most popular formulations of Nutricote into greenhouse root substrates
- Materials, rates necessary to lower the pH level of greenhouse potting substrate 0.5 to 1.0 units
- Approximate amount of materials required to change pH of potting mixes
- Dilution/conversion chart for various chemical growth regulators
- Pre-plant fertilizer sources and rates of application
- Cornell Peat-lite Mix A for seedlings, bedding plants and potted plants
- Number of pots per bushel and per cubic yard of soil mix
- Number of nursery containers that can be filled from 1 yd3 of soil mix
- Coverage estimates for perlite, peat, topsoil and straw
- Plant spacing guide (greenhouse)
- Plant spacing guide (field/orchard)
- Estimated number of plants to fill 100 ft2 bed area for square (row) and triangular (equilateral) planting patterns using 4 to 14-in. spacing distances
- Number of bedding/groundcover plants required at various spacing for landscape planting
- Number of plants per acre at various spacings
- Times required to mow or trim lawn areas
- Volume of water delivered – by size of hose
- Cubic yards of soil needed at various depths and areas
- Areas covered in square feet at various depths
- Temperature conversion
- Formulas for calculating greenhouse volume
- Formulas for calculating variously shaped areas
Pesticide and fertilizer recommendations often are made on a pounds-per-acre or tons-per-acre basis for field production. However, greenhouse and nursery operators, landscape professionals and orchardists often must convert these recommendations to smaller areas, such as row feet or square feet per tree or per pot. Pints, cups, ounces, tablespoons and teaspoons often are the common units of measure. Metric units of measure can further complicate conversion.
This publication is designed to help growers make these calculations and conversions and to provide other data useful in the management, planning and operation of horticultural enterprises. A number of formulas for calculating fertilizer application rates on a parts-per-million basis are given. Tables for fertilizer injector calibration using a conductivity meter, as well as pre-plant application rates for various soil mix components and amendments, also are provided. A brief explanation of how each table is used is provided.
Tables 1 through 3 help determine equivalent measures for liquid (volume) or dry (weight) chemical substances and also converting metric to English units.
Tables 4 through 7 help determine correct application rates for various pesticides.
Tables 8 through 9 help determine the correct application rates for fertilizers when nutrition recommendations are based on fertilizer weight.
Tables 10 through 14 help determine the correct application rates for fertilizers with various analysis when nutrition recommendations are based on parts per million and fertilizer injectors are used to deliver liquid plant fertilizer. Table 12 is designed to help growers calibrate their injectors.
| Table 12. Injector calibration with a conductivity meter1 | |||||||
|---|---|---|---|---|---|---|---|
| B. Peters Mixed Soluble Fertilizer Analysis | |||||||
| ppm Nitrogen | 20-20-20 20-19-18 |
20-10-15 | 20-5-30 | 25-5-20 | 25-10-10 30-10-10 |
5-11-26 Hydrosol |
15-16-17 15-11-29 15-20-25 |
| 50 | 0.23 | 0.31 | 0.22 | 0.12 | 0.09 | 1.00 | 0.32 |
| 75 | 0.34 | 0.47 | 0.33 | 0.18 | 0.14 | 1.50 | 0.48 |
| 100 | 0.45 | 0.62 | 0.44 | 0.24 | 0.18 | 2.00 | 0.65 |
| 125 | 0.56 | 0.78 | 0.56 | 0.30 | 0.23 | 2.50 | 0.82 |
| 150 | 0.68 | 0.93 | 0.69 | 0.36 | 0.27 | 3.00 | 1.00 |
| 175 | 0.79 | 1.09 | 0.81 | 0.43 | 0.32 | 3.50 | 1.20 |
| 200 | 0.90 | 1.24 | 0.94 | 0.51 | 0.36 | 4.00 | 1.40 |
| 225 | 1.01 | 1.40 | 1.07 | 0.57 | 0.41 | 4.50 | 1.56 |
| 250 | 1.13 | 1.55 | 1.20 | 0.62 | 0.47 | 5.00 | 1.72 |
| 275 | 1.24 | 1.71 | 1.32 | 0.71 | 0.51 | 5.50 | 1.91 |
| 300 | 1.35 | 1.86 | 1.43 | 0.80 | 0.54 | 6.00 | 2.10 |
| 350 | 1.58 | 2.17 | 1.66 | 0.92 | 0.64 | 6.50 | 2.45 |
| 400 | 1.80 | 2.48 | 1.90 | 1.04 | 0.74 | 7.00 | 2.80 |
| 450 | 2.03 | 2.79 | 2.15 | 1.18 | 0.85 | 7.50 | 3.15 |
| 500 | 2.25 | 3.10 | 2.40 | 1.32 | 0.96 | 8.00 | 3.50 |
| 550 | 2.48 | 3.41 | 2.61 | 1.45 | 1.06 | – | 3.84 |
| 600 | 2.70 | 3.72 | 2.82 | 1.58 | 1.16 | – | 4.18 |
| 650 | 2.93 | 4.03 | 3.03 | 1.71 | 1.26 | – | 4.52 |
| 700 | 3.15 | 4.34 | 3.24 | 1.84 | 1.36 | – | 4.80 |
| 750 | 3.38 | 4.65 | 3.45 | 1.98 | 1.46 | – | 5.20 |
| 800 | 3.60 | 4.96 | 3.66 | 2.11 | 1.56 | – | 5.54 |
| 850 | 3.83 | 5.27 | 3.87 | 2.24 | 1.66 | – | 5.88 |
| 900 | 4.05 | 5.58 | 4.08 | 2.37 | 1.76 | – | 6.22 |
| 950 | 4.28 | 5.89 | 4.29 | 2.50 | 1.86 | – | 6.56 |
| 1000 | 4.50 | 6.20 | 4.5 | 2.63 | 1.96 | – | 6.90 |
| 1Adapted from Grace Horticultural Products. W.1 R. Grace & Co. Cambridge, Massachusetts 02140. NOTES: 1) For use with meters in millimhos with Peters® Fertilizer formulations. 2) These readings are made with distilled water. 3) Test your plain irrigation water first and subtract that reading from the fertilizer-injected water. For example, your water test indicates 0.2 mmhos and you are applying 200 ppm N with 15-15-15 fertilizer. Your calibration reading is 1.30 – 0.2 = 1.10 mmhos. |
|||||||
| Table 12. Injector calibration with a conductivity meter1 | |||||||
|---|---|---|---|---|---|---|---|
| B. Peters Mixed Soluble Fertilizer Analysis (cont.) | |||||||
| ppm Nitrogen | 15-15-15 | 15-10-30 | 15-30-15 | 15-0-15 | 16-4-12 | 21-7-7 Acid |
21-7-7 Neutral |
| 50 | 0.30 | 0.32 | 0.31 | 0.36 | 0.32 | 0.28 | 0.21 |
| 75 | 0.46 | 0.51 | 0.47 | 0.55 | 0.48 | 0.42 | 0.32 |
| 100 | 0.62 | 0.70 | 0.62 | 0.74 | 0.64 | 0.56 | 0.42 |
| 125 | 0.79 | 0.87 | 0.78 | 0.94 | 0.81 | 0.70 | 0.53 |
| 150 | 0.96 | 1.50 | 0.93 | 1.15 | 0.98 | 0.84 | 0.63 |
| 175 | 1.13 | 1.23 | 1.09 | 1.35 | 1.14 | 0.98 | 0.74 |
| 200 | 1.30 | 1.41 | 1.24 | 1.55 | 1.31 | 1.12 | 0.84 |
| 225 | 1.47 | 1.59 | 1.40 | 1.72 | 1.47 | 1.26 | 0.95 |
| 250 | 1.65 | 1.78 | 1.55 | 1.90 | 1.62 | 1.40 | 1.05 |
| 275 | 1.82 | 1.95 | 1.71 | 2.09 | 1.81 | 1.54 | 1.16 |
| 300 | 1.98 | 2.12 | 1.86 | 2.28 | 2.00 | 1.68 | 1.26 |
| 350 | 2.31 | 2.45 | 2.17 | 2.64 | 2.29 | 1.96 | 1.47 |
| 400 | 2.65 | 2.78 | 2.48 | 3.00 | 2.58 | 2.24 | 1.68 |
| 450 | 2.98 | 3.12 | 2.79 | 3.34 | 2.93 | 2.52 | 1.89 |
| 500 | 3.25 | 3.46 | 3.10 | 3.68 | 3.28 | 2.80 | 2.10 |
| 550 | 3.55 | 3.76 | 3.41 | 3.98 | 3.57 | 3.08 | 2.31 |
| 600 | 3.85 | 4.06 | 3.72 | 4.28 | 3.86 | 3.36 | 2.52 |
| 650 | 4.15 | 4.36 | 4.03 | 4.58 | 4.15 | 3.64 | 2.73 |
| 700 | 4.45 | 4.66 | 4.34 | 4.88 | 4.44 | 3.92 | 2.94 |
| 750 | 4.75 | 4.95 | 4.65 | 5.20 | 4.72 | 4.20 | 3.15 |
| 800 | 5.05 | 5.25 | 4.96 | 5.50 | 4.98 | 4.48 | 3.36 |
| 850 | 5.35 | 5.55 | 5.27 | 5.80 | 5.24 | 4.76 | 3.57 |
| 900 | 5.65 | 5.85 | 5.58 | 6.10 | 5.50 | 5.04 | 3.78 |
| 950 | 5.95 | 6.15 | 5.89 | 6.40 | 5.76 | 5.32 | 3.99 |
| 1000 | 6.25 | 6.45 | 6.20 | 6.70 | 6.00 | 5.60 | 4.20 |
| 1Adapted from Grace Horticultural Products. W.1 R. Grace & Co. Cambridge, Massachusetts 02140. NOTES: 1) For use with meters in millimhos with Peters® Fertilizer formulations. 2) These readings are made with distilled water. 3) Test your plain irrigation water first and subtract that reading from the fertilizer-injected water. For example, your water test indicates 0.2 mmhos and you are applying 200 ppm N with 15-15-15 fertilizer. Your calibration reading is 1.30 – 0.2 = 1.10 mmhos. |
|||||||
| Table 13A. Parts per million of desired nutrient to ounces of fertilizer carrier in 100 gallons of water and vice versa.1 (cont) | ||||||
|---|---|---|---|---|---|---|
| Ounces of Fertilizer Carrier in 100 Gallons |
Percentage of Desired Nutrient in Fertilizer Carrier | |||||
| 33 | 44 | 45 | 53 | 60 | 62 | |
| 1 | 24.7 | 32.9 | 33.7 | 39.7 | 44.9 | 46.4 |
| 2 | 49.4 | 65.9 | 67.4 | 79.3 | 89.8 | 92.0 |
| 3 | 74.1 | 98.8 | 101.0 | 117.0 | 134.7 | 139.2 |
| 4 | 98.8 | 131.7 | 134.7 | 158.7 | 179.6 | 185.6 |
| 6 | 148.2 | 197.6 | 202.1 | 238.0 | 269.4 | 278.4 |
| 8 | 197.6 | 263.4 | 269.4 | 317.3 | 359.2 | 371.2 |
| 16 | 395.2 | 526.9 | 538.9 | 634.6 | 718.5 | 742.4 |
| 24 | 592.7 | 790.3 | 808.3 | 952.0 | 1077.7 | 1113.6 |
| 32 | 790.3 | 1053.7 | 1077.7 | 1269.3 | 1436.9 | 1484.8 |
| 40 | 987.9 | 1317.2 | 1347.1 | 1586.6 | 1796.2 | 1856.1 |
| 48 | 1185.5 | 1580.6 | 1616.5 | 1903.9 | 2155.4 | 2227.2 |
| 56 | 1383.0 | 1844.0 | 1886.0 | 2221.2 | 2514.6 | 2598.4 |
| 64 | 1580.6 | 2107.5 | 2155.4 | 2538.6 | 2873.9 | 2969.7 |
| 1From Nelson, P. V. 1998. Greenhouse Operations and Management, 5th ed. Published by Prentice Hall, Inc. Reprinted with permission. | ||||||
| Table 13B. Parts per million of desired nutrient to ounces of fertilizer carrier in 100 gallons of water and vice versa.1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Grams of Fertilizer Carrier in 1 Liter |
PPM of Desired Nutrient in Fertilizer Carrier | |||||||
| 12 | 13 | 14 | 15.5 | 16 | 20 | 20.5 | 21 | |
| 0.1 | 12 | 13 | 14 | 16 | 16 | 20 | 20.5 | 21 |
| 0.2 | 24 | 26 | 28 | 31 | 3 | 40 | 41.0 | 42 |
| 0.3 | 36 | 39 | 42 | 47 | 48 | 60 | 61.5 | 63 |
| 0.4 | 48 | 52 | 56 | 62 | 64 | 80 | 82.0 | 84 |
| 0.6 | 72 | 78 | 84 | 93 | 96 | 120 | 123.0 | 126 |
| 0.8 | 96 | 104 | 112 | 124 | 128 | 160 | 164.0 | 168 |
| 1.0 | 120 | 130 | 140 | 155 | 160 | 200 | 205.0 | 210 |
| 1.5 | 180 | 195 | 210 | 233 | 240 | 300 | 307.0 | 315 |
| 2.0 | 240 | 260 | 280 | 310 | 320 | 400 | 410.0 | 420 |
| 2.5 | 300 | 325 | 350 | 388 | 400 | 500 | 512.5 | 525 |
| 3.0 | 360 | 390 | 420 | 465 | 480 | 600 | 615.0 | 630 |
| 3.5 | 420 | 455 | 490 | 543 | 560 | 700 | 717.5 | 735 |
| 4.0 | 480 | 520 | 560 | 620 | 640 | 800 | 820.0 | 840 |
| 1From Nelson, P. V. 1998. Greenhouse Operations and Management, 5th ed. Published by Prentice Hall, Inc. Reprinted with permission. | ||||||||
| Table 13B. Parts per million of desired nutrient to grams of fertilizer carrier in 1 liter water and vice versa.1 (cont) | ||||||
|---|---|---|---|---|---|---|
| Grams of Fertilizer Carrier in 1 Liter |
PPM of Desired Nutrient in Fertilizer Carrier | |||||
| 33 | 44 | 45 | 53 | 60 | 62 | |
| 0.1 | 33 | 44 | 45 | 53 | 60 | 62 |
| 0.2 | 66 | 88 | 90 | 106 | 120 | 124 |
| 0.3 | 99 | 132 | 135 | 159 | 180 | 186 |
| 0.4 | 132 | 176 | 180 | 212 | 240 | 248 |
| 0.6 | 198 | 264 | 270 | 318 | 360 | 372 |
| 0.8 | 264 | 352 | 360 | 424 | 480 | 496 |
| 1.0 | 330 | 440 | 450 | 530 | 600 | 620 |
| 1.5 | 495 | 660 | 675 | 795 | 900 | 930 |
| 2.0 | 660 | 880 | 900 | 1060 | 1200 | 1240 |
| 2.5 | 825 | 1100 | 1125 | 1325 | 1500 | 1550 |
| 3.0 | 990 | 1320 | 1350 | 1590 | 1800 | 1860 |
| 3.5 | 1155 | 1540 | 1575 | 1855 | 2100 | 2170 |
| 4.0 | 1320 | 1760 | 1800 | 2120 | 2400 | 2480 |
| 1From Nelson, P. V. 1998. Greenhouse Operations and Management, 5th ed. Published by Prentice Hall, Inc. Reprinted with permission. | ||||||
Table 15 is designed to help growers decide which acid to add and in what quantities to acidify their irrigation water.
| Table 15. Various acids to add to irrigation water for acidification. | ||||||
| Note: The table is an example from a website called AlkCalc, available at https://e-gro.org/alkcalc/dist/index.html. It is an acidification analysis done on a water sample with a starting pH of 8.0 and alkalinity of 200 ppm CaCO3 acidified to an end point pH of 5.8. For your specific water sample, follow the directions on the website. You will need to obtain a water report on your irrigation water prior to using AlkCalc. You will need to know the water pH and alkalinity of your sample and have an idea about what end-point pH you want to obtain after acidification. The wesbite also gives you information about the cost of the acidification treatment. | ||||||
| ALTERNATIVE ACIDS TO ADD TO IRRIGATION WATER | ||||||
|---|---|---|---|---|---|---|
| Amounts | Acids | |||||
| Phosphoric Acid (75%) | Phosphoric Acid (85%) | Sulfuric Acid (35%) | Sulfuric Acid (93%) | Nitric Acid (61.4%) | Nitric Acid (67%) | |
| For Small Volumes | ||||||
| ml per liter | 0.253 | 0.207 | 0.348 | 0.087 | 0.234 | 0.209 |
| fl oz per gallon | 0.032 | 0.027 | 0.044 | 0.011 | 0.030 | 0.027 |
| ml per gallon | 0.956 | 0.785 | 1.316 | 0.330 | 0.884 | 0.793 |
| For a 1:100 Injector | ||||||
| fl oz per gallon (conc.) | 3.23 | 2.65 | 4.45 | 1.12 | 2.99 | 2.68 |
| ml per gallon (conc.) | 95.63 | 78.47 | 131.59 | 32.98 | 88.40 | 79.28 |
| For a 1:128 Injector | ||||||
| fl oz per gallon (conc.) | 4.14 | 3.40 | 5.70 | 1.43 | 3.83 | 3.43 |
| ml per gallon (conc.) | 122.41 | 100.44 | 168.44 | 42.22 | 113.16 | 101.48 |
| For a 1:200 Injector | ||||||
| fl oz per gallon (conc.) | 6.47 | 5.31 | 8.90 | 2.23 | 5.98 | 5.36 |
| ml per gallon (conc.) | 191.27 | 156.94 | 263.19 | 65.97 | 176.81 | 158.56 |
| NUTRIENTS ADDED BY EACH TYPE OF ACID | ||||||
| Nutrients Added | Phosphorus | Phosphorus | Sulfur | Sulfur | Nitrogen | Nitrogen |
| Amount Added (ppm) | 94.6 | 94.6 | 50.3 | 50.3 | 43.7 | 43.7 |
| Use the information above for modifying your fertility program. | ||||||
Tables 16 through 20 help determine which fertilizers to use based on chemical analysis, reaction in substrate, longevity in substrate (slow release fertilizers), and incorporation rates for some popular slow release fertilizers. Tables 17 and 18 are specifically designed to provide detailed information on fertilizer calculations, which also aid determine correct application rates.
Using Chemicals
1)
mg of fertilizer source/liter of water = (ppm)(formula weight) (atomic weight of element)(number of units in formula of fertilizer source)
2)
ppm = (mg of fertilizer/liter of water)(atomic weight of element)(number of units of element in formula of fertilizer source) (formula weight of fertilizer source)
3) to convert mg/L to lb/100 gallons, multiply milligrams by 0.0008344
4) to convert lb/100 gallons to mg/L, divide pounds by 0.0008344
EXAMPLE: How many pounds of potassium sulfate (K2SO4) need to be dissolved in 100 gallons of water to make 100 ppm K solution. Get the formula weight of potassium sulfate (K2SO4) and the atomic weight of potassium from Table 14. Then:
1) mg of K2SO4 / liter of water = (100 x 174.2) ÷ (39.1 x 2) = 222.8 mg/L
2) 222.8 mg/L x 0.00083440 = 0.186 lb potassium sulfate/100 gallons
Using Premixed Fertilizers
1)
mg of mixed fertilizer/liter of water = (ppm of N desired)(100) (% N in fertilizer)
2)
ppm of P = (mg of mixed fertilizer/liter of water)(% P2O5)(0.4366) 100
3)
ppm of K = (mg of mixed fertilizer/liter of water) (% K2O) (0.8301) 100
4)
mg of mixed fertilizer/liter of water = (ppm of P desired)(100) (% P2O5)(0.4366)
5)
mg of mixed fertilizer/liter of water = (ppm of k desired)(100) (% K2O) (0.8301)
6)
mg of mixed fertilizer/liter of water = (mg of mixed fertilizer/liter of water)(% N) 10
| Table 18. Miscellaneous conversions used in fertilizer calculations | ||
|---|---|---|
| 1 millimeter or cubic centimeter of water weighs 1 gram | ||
| 1 liter of water weighs 1 kilogram | ||
| 1 gallon of water weighs 8.34 lb | ||
| 1 part per million (ppm) | = 0.0001% = 1 milligram/liter =0.013 ounces in 100 gallons of water |
|
| 1% | = 10,000 ppm = 10 grams per liter = 10,000 grams per kilogram = 1.33 ounces by weight per gallon of water = 8.34 lb per 100 gallons of water |
|
| 0.1% | = 1000 ppm | = 1000 milligrams per liter |
| 0.01% | = 100 ppm | = 100 milligrams per liter |
| 0.001% | = 10 ppm | = 10 milligrams per liter |
| 0.0001% | = 1 ppm | = 1 milligram per liter |
| Approximate weight-volume measurements for making small volumes of water soluble fertilizers | ||
| 1 cup | = 8 oz or 0.5 lb of fertilizer | |
| 2 cups | = 1 lb of fertilizer | |
| 1 tablespoon | = 0.5 oz of fertilizer | |
| 2 tablespoons | = 1 oz of fertilizer | |
| Useful conversions | ||
| 1 ton/acre | = 20.8 g/square foot | |
| 1 ton/acre | = 1 lb/21.78 square feet | |
| 1 g/square foot | = 96 lb/acre | |
| 1 lb/acre | = 0.0104 g/square foot | |
| 100 lb/acre | = 0.2296 lb/100 square feet | |
| grams/square foot x 96 | = lb/acre | |
| lb/square foot x 43,560 | = lb/acre | |
| 100 square feet | = 1/435.6 or 0.002296 acres | |
| Weight conversions from lb/acre to weight/100 square feet | ||
| lb/acre | amount applied/100 square feet | |
| 100 | 3.7 oz | |
| 200 | 7.4 oz | |
| 300 | 11.1 oz | |
| 400 | 14.8 oz | |
| 500 | 1 lb 2.5 oz | |
| 600 | 1 lb 6 oz | |
| 700 | 1 lb 10 oz | |
| 800 | 1 lb 13 oz | |
| 900 | 2 lb 1 oz | |
| 1000 | 2 lb 5 oz | |
| 2000 | 4 lb 10 oz | |
| Percent to Ratio Conversion | ||
| 2.0% | 1:50 | |
| 1.5% | 1:67 | |
| 1.0% | 1:100 | |
| 0.9% | 1:111 | |
| 0.8% | 1:128 | |
| 0.7% | 1:143 | |
| 0.6% | 1:167 | |
| 0.5% | 1:200 | |
| 0.4% | 1:250 | |
| 0.3% | 1:333 | |
| 0.2% | 1:500 | |
| Table19. Osmocote® controlled-release fertilizers and their release periods1 | ||
|---|---|---|
| Analysis | Longevity2 (months) | Product Name |
| 14-14-14 | 3-4 | Osmocote®3 |
| 19-6-12 | 3-4 | Osmocote®3 |
| 13-13-13 | 8-9 | Osmocote®3 |
| 18-6-12 | 8-9 | Osmocote®3 Fast Start |
| 18-6-12 | 8-9 | Osmocote®3 |
| 17-7-12 | 12-14 | Osmocote®3 |
| 15-9-12 | 3-4 | Osmocote® Plus |
| 15-9-12 | 5-6 | Osmocote® Plus |
| 15-9-12 | 8-9 | Osmocote® Plus |
| 15-9-12 | 12-14 | Osmocote® Plus |
| 15-9-12 | 14-16 | Osmocote® Plus |
| 16-8-12 | 8-9 | Osmocote® Plus Minors Tablets |
| 19-5-8 + Minors | 8-9 | Osmocote® Pro with Poly-S |
| 19-5-9 + Minors | 12-14 | Osmocote® Pro with Poly-S |
| 20-5-8 + Minors | 8-9 | Osmocote® Pro with Poly-S |
| 24-4-8 | 8-9 | Osmocote® Pro with Resin Coated Urea |
| 24-4-7 | 12-14 | Osmocote® Pro with Resin Coated Urea |
| 24-4-6 | 14-16 | Osmocote® Pro with Resin Coated Urea |
| 21-4-7 w/ Mg & Fe | 8-9 | Osmocote® Pro with Resin Coated Urea |
| 21-3-7 w/ Mg & Fe | 12-14 | Osmocote® Pro with Resin Coated Urea |
| 22-4-9 + Minors | 5-6 | Osmocote® Pro with Resin Coated Urea |
| 22-4-8 + Minors | 8-9 | Osmocote® Pro with Resin Coated Urea |
| 22-4-7 + Minors | 12-14 | Osmocote® Pro with Resin Coated Urea |
| 22-4-6 + Minors | 14-16 | Osmocote® Pro with Resin Coated Urea |
| 20-4-9 | 8-9 | Osmocote® Pro with Methylene Urea and Ureaform |
| 20-4-8 | 12-14 | Osmocote® Pro with Methylene Urea and Ureaform |
| 23-4-8 + Minors | 14-16 | Osmocote® Pro + ScottKote™ |
| 19-7-10 + Fe | 3-4 | Osmocote® Pro with Uncoated NPK and Iron |
| 18-7-10 + Fe | 8-9 | Osmocote® Pro with Uncoated NPK and Iron |
| 17-7-10 + Fe | 12-14 | Osmocote® Pro with Uncoated NPK and Iron |
| 13-10-13 | 5-6 | Osmocote® Pro with IBDU and Minors |
| 15-10-10 | 8-9 | Osmocote® Pro with IBDU and Minors |
| 18-8-8 | 8-9 | Osmocote® Pro with IBDU and Minors |
| 20-4-8 | 8-9 | Osmocote® Pro with IBDU and Minors |
| 18-5-9 | 12-14 | Osmocote® Pro with IBDU and Minors |
| 17-6-12 + Minors | 3-4 | Sierra® Tablets |
| 17-6-10 + Minors | 8-9 | Sierra® Tablets |
| 1 From the Scotts Company and Subsidiaries, Marysville, OH 43041. 2 At an average root substrate temperature of 70 °F (21 °C). 3 Six trace elements plus magnesium. |
||
Tables 21 through 22 are designed to assist growers in correcting the pH of the growing substrate.
Table 23 will help when applying various plant growth regulators.
| Table 23B. Dilution/conversion chart for CYCOCEL (11.8% active ingredient). | |||||
|---|---|---|---|---|---|
| Spray | |||||
| Spray Solution (ppm) | Fluid Ounces per Gallon of Final Solution | Milliliters per Gallon of Final Solution | Milliliters per Liter of Final Solution |
||
| 1,000 | 1.08 | 32.08 | 8.47 | ||
| 1,500A | 1.63 | 48.12 | 12.71 | ||
| 2,000 | 2.17 | 64.16 | 16.95 | ||
| 2,500 | 2.71 | 80.20 | 21.19 | ||
| 3,000B | 3.25 | 96.24 | 25.42 | ||
| 5,000 | 5.42 | 160.40 | 42.37 | ||
| Drench | |||||
| Dose (Milligrams per 6-in. Pot) | Drench Volume per 6-in. Pot* (Fluid Ounces) |
ppm solution | Fluid Ounces per Gallon of Final Solution | Milliliters per Gallon of Final Solution | Milliliters per Liter of Final Solution |
| 355 | 6 | 2,000 | 2.17 | 64.18 | 16.95 |
| 532 | 6 | 3,000B | 3.25 | 96.18 | 25.42 |
| 710 | 6 | 4,000 | 4.34 | 128.36 | 33.90 |
| Adapted from Hammer, P.A. 1992. ACommonly referred to as 1:80. BCommonly referred to as 1:40. *2 fl oz/2.25- to 3-in. pot; 3 fl oz/4-in. pot; 4 fl oz/5-in. pot; 8 fl oz/8-in. pot. |
|||||
| Table 23C. Dilution/conversion chart for B-NINE WSG (85% active ingredient). | |||
|---|---|---|---|
| Spray | |||
| Spray Solution (ppm) | Ounces per Gallon of Final Solution | Grams per Gallon of Final Solution | Grams per Liter of Final Solution |
| 1,000 | 0.16 | 4.45 | 1.18 |
| 2,500 | 0.39 | 11.13 | 2.94 |
| 5,000 | 0.79 | 22.26 | 5.88 |
| 7,500 | 1.18 | 33.40 | 8.82 |
| Adapted from Hammer, P.A. 1992. | |||
| Table 23D. Dilution/conversion chart for BONZI (0.4% active ingredient). | |||||
|---|---|---|---|---|---|
| Spray | |||||
| Spray Solution (ppm) | Fluid Ounces per Gallon of Final Solution | Milliliters per Gallon of Final Solution | Milliliters per Liter of Final Solution | ||
| 1 | 0.032 | 0.95 | 0.25 | ||
| 3 | 0.096 | 2.84 | 0.75 | ||
| 5 | 0.160 | 4.73 | 1.25 | ||
| 10 | 0.320 | 9.46 | 2.50 | ||
| 15 | 0.480 | 14.20 | 3.75 | ||
| 25 | 0.800 | 23.66 | 6.25 | ||
| 45 | 1.440 | 42.59 | 11.25 | ||
| 60 | 1.920 | 56.78 | 15.00 | ||
| 90 | 2.880 | 85.17 | 22.50 | ||
| Drench | |||||
| Dose (Milligrams per 6-in. Pot) | Drench Volume per 6-in. Pot* (Fluid Ounces) |
ppm | Fluid Ounces per Gallon of Final Solution | Milliliters per Gallon of Final Solution | Milliliters per Liter of Final Solution |
| 0.1 | 4 | 0.85 | 0.03 | 0.8 | 0.21 |
| 0.2 | 4 | 1.69 | 0.05 | 1.6 | 0.42 |
| 0.5 | 4 | 4.23 | 0.14 | 4.0 | 1.06 |
| 1.0 | 4 | 8.45 | 0.27 | 8.0 | 2.11 |
| 1.9 | 4 | 16.06 | 0.51 | 15.2 | 4.02 |
| Adapted from Hammer, P.A. 1992. * 2 fl oz/4-in. pot; 3 fl oz/5-in. pot; 10 fl oz/8-in. pot. |
|||||
| Table 23E. Dilution/conversion chart for SUMAGIC (0.055% active ingredient). | |||||
|---|---|---|---|---|---|
| Spray | |||||
| Spray Solution (ppm) | Fluid Ounces per Gallon of Final Solution | Milliliters per Gallon of Final Solution | Milliliters per Liter of Final Solution | ||
| 1 | 0.26 | 7.57 | 2 | ||
| 3 | 0.77 | 22.71 | 6 | ||
| 5 | 1.28 | 37.85 | 10 | ||
| 10 | 2.56 | 75.71 | 20 | ||
| 15 | 3.84 | 113.56 | 30 | ||
| 25 | 6.40 | 189.27 | 50 | ||
| 30 | 7.68 | 227.12 | 60 | ||
| 50 | 12.80 | 378.54 | 100 | ||
| Drench | |||||
| Dose (Milligrams per 6-in. Pot) | Drench Volume per 6-in. Pot* (Fluid Ounces) |
ppm | Fluid Ounces per Gallon of Final Solution | Milliliters per Gallon of Final Solution | Milliliters per Liter of Final Solution |
| 0.02 | 4 | 0.17 | 0.04 | 1.28 | 0.34 |
| 0.03 | 4 | 0.25 | 0.06 | 1.92 | 0.51 |
| 0.04 | 4 | 0.34 | 0.09 | 2.56 | 0.68 |
| 0.05 | 4 | 0.42 | 0.11 | 3.20 | 0.85 |
| 0.06 | 4 | 0.51 | 0.13 | 3.84 | 1.01 |
| 0.09 | 4 | 0.76 | 0.19 | 5.76 | 1.52 |
| 0.12 | 4 | 1.01 | 0.26 | 7.68 | 2.03 |
| 0.20 | 4 | 1.69 | 0.43 | 12.80 | 3.38 |
| Adapted from Hammer, P.A. 1992. * 2 fl oz/4-in. pot; 3 fl oz/5-in. pot; 10 fl oz/8-in. pot. |
|||||
| Table 23F. Dilution/conversion chart for FLOREL (3.9% active ingredient). | |||
|---|---|---|---|
| Spray | |||
| Spray Solution (ppm) | Fluid Ounces per Gallon of Final Solution | Milliliters per Gallon of Final Solution | Milliliters per Liter of Final Solution |
| 300 | 0.97 | 28.72 | 7.59 |
| 325 | 1.05 | 331.11 | 8.22 |
| 500 | 1.62 | 47.86 | 12.64 |
| 750 | 2.43 | 28.89 | 18.97 |
| 975 | 3.16 | 93.34 | 24.66 |
| 1,000 | 3.24 | 95.73 | 25.29 |
| Adapted from Hammer, P.A. 1992. | |||
| Table 23G. Dilution/conversion chart for PRO-GIBB (4% active ingredient). | |||
|---|---|---|---|
| Spray | |||
| Spray Solution (ppm) | Fluid Ounces per Gallon of Final Solution | Milliliters per Gallon of Final Solution | Milliliters per Liter of Final Solution |
| 2.5 | 0.008 | 0.24 | 0.06 |
| 5.0 | 0.016 | 0.47 | 0.13 |
| 100.0 | 0.320 | 9.46 | 2.50 |
| 250.0 | 0.800 | 23.66 | 6.25 |
| 300.0 | 0.960 | 28.39 | 7.50 |
| 500.0 | 1.600 | 47.31 | 12.50 |
| Adapted from Hammer, P.A. 1992. | |||
| Table 23H. Dilution/conversion chart for FASCINATION. | |||
|---|---|---|---|
| Spray | |||
| ppm BA/GA | Fluid Ounces per Gallon of Final Solution | Milliliters per Gallon of Final Solution | Milliliters per Liter of Final Solution |
| 1/1 | 0.007 | 0.2 | 0.06 |
| 5/5 | 0.04 | 1.1 | 0.3 |
| 10/10 | 0.07 | 2.1 | 0.6 |
| 25/25 | 0.18 | 5.3 | 1.4 |
| 50/50 | 0.36 | 10.5 | 2.8 |
| 75/75 | 0.53 | 15.8 | 4.2 |
| 100/100 | 0.71 | 21.0 | 5.5 |
| Adapted from Hammer, P.A. 1992. | |||
Tables 24 through 25 are designed to assist growers who desire to prepare their own substrate mix.
Tables 29 through 30 help determine correct spacing and number of plants at each spacing for both greenhouse and field situations.
Formulas for calculating greenhouse volume
These formulas are helpful in determining heating and cooling costs for greenhouses.
For the following formulas:
L = length
W = width
W1 = width of short span
W2 = width of long span
He = height from floor to eave
Hr = height from eave to top
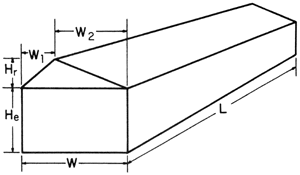 Uneven-span greenhouses
Uneven-span greenhousesFigure 1-A. Formula for calculating uneven-span greenhouse volume.
Greenhouse volume in cubic feet = [(W x He) + ([W1 x Hr] ÷ 2) + ([W2 x Hr] ÷ 2)] x L
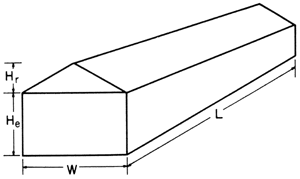 Even-span greenhouses
Even-span greenhousesFigure 1-B. Formula for calculating even-span greenhouse volume.
Greenhouse volume in cubic feet = [(W x He) + ([W x Hr] ÷ 2)] x L
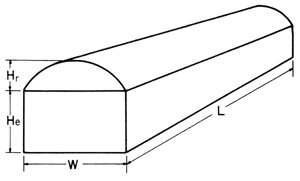 Quonset structures
Quonset structuresFigure 1-C. Formula for calculating quonset greenhouse volume.
Greenhouse volume in cubic feet = [(W x He) + ([3.14 x Hr²] ÷ 2)] x L
Acknowledgments
The authors wish to acknowledge the following sources, from which certain tables were adapted for use in this publication.
Bailey, D. A. (1996). Alkalinity, pH and Acidification. In D. Reed (Ed.), A Grower’s Guide to Water, Media, and Nutrition for Greenhouse Crops. Ball Publishing.
Bailey, D. A., & Powell, M. A. (1999). Installation and maintenance of landscape bedding plants (Horticulture Information Leaflet 555). N.C. State University and N.C. A&T State University Cooperative Extension. https://digital.ncdcr.gov/Documents/Detail/installation-and-maintenance-of-landscape-bedding-plants/3692207
Ball, V. (Ed.). Ball RedBookBall Publishing.
Cavins, T. J., Gibson, J. L., Whipker, B. E., & Fonteno, W. C. (2000, December). pH and EC meters — Tools for substrate analysis (Research Report Florex.001). North Carolina State University. https://fertdirtsquirt.com/pdf/PHECmeters.pdf
Conover, C. A., & Poole, R. T. (1984). Light and fertilizer recommendations for production of acclimatized potted foliage plants. Foliage Digest, 7(8), 1–6.
Cornell University. (1977). Cornell recommendations for commercial floricultural crops: Part 1, cultural practices and production programs. https://digital.library.cornell.edu/catalog/chla7134041_8300_001
Holcomb, E. J. (Ed.). (1994). Ball Publishing.
Hummert International. (1999). Hummert’s helpful hints, 1999–2000 ed.
Nelson, P. V. (1998). Greenhouse operation and management (5th ed.). Prentice Hall.
Ohio Florist Association Services, Inc. (1999). Tips on growing bedding plants, 4th ed.
Ohio Florist Association Services, Inc. (1992). Tips on the use of chemical growth regulators on floriculture crops.
University of California Cooperative Extension Service. (n.d.). Tons to teaspoons (Pubication No. L2285).
Whipker, B. E., Bailey, D. A., Nelson, P. V., Fonteno, W. C., & Hammer, P. A. (n.d.). Greenhouse media lab acid addition calculator to control alkalinity in irrigation water. Cooperative Extension Services of the Northeast States.
DISCLAIMER: Trade named products listed does not imply endorsement over similar products, which may also be available.








